Instructions
Ready to get started with this activity? To keep track of your progress, check off the instructions for each step below as they are completed. Make sure to check the box of the last step when you’re done to receive congratulations for your completed activity!
Consider this:
Some homes with solar panels create more electricity than they need. This usually happens during the day when the occupants are away at school or work. Electricity is still needed in the home for the heater, air conditioning, refrigerator, hot water tank, and so much more – but the demand for electricity is generally lower without people inside a building. Because the extra electricity isn’t needed, it can return to the grid for someone else to use. Clark Public Utilities buys electricity for the same amount it’s sold. Other homes have batteries to help store the extra energy to use later when they need it. Engineers at a variety of companies are eager to make the batteries smaller and more affordable.
Ready to make a battery for just a few dollars? Voltaic batteries come in all shapes and sizes and convert chemical energy into electrical energy. In this activity, we will make a battery by using the chemical energy stored in lemons. Two different metals will help make a current to light up a small light emitting diode (LED).
- (4) Lemons
- (4) Zinc-galvanized nails
- (4) Pennies
- Knife
- (5) Alligator clip wires (colors do not need to match)
- LED

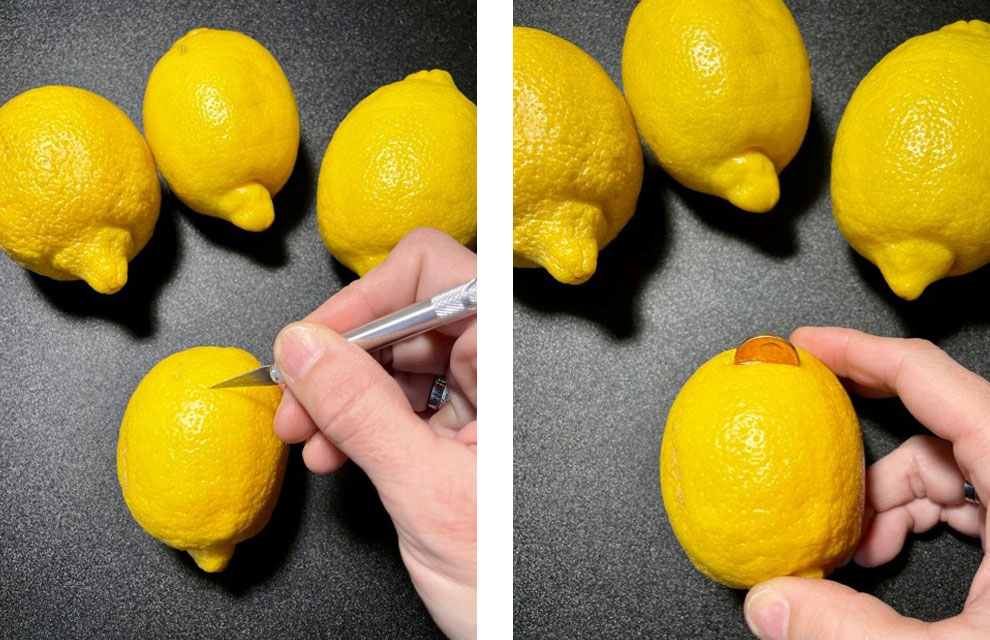
- Carefully cut a penny-sized slit in each of the lemons.
- Insert the penny halfway into the slit – do this to each lemon.
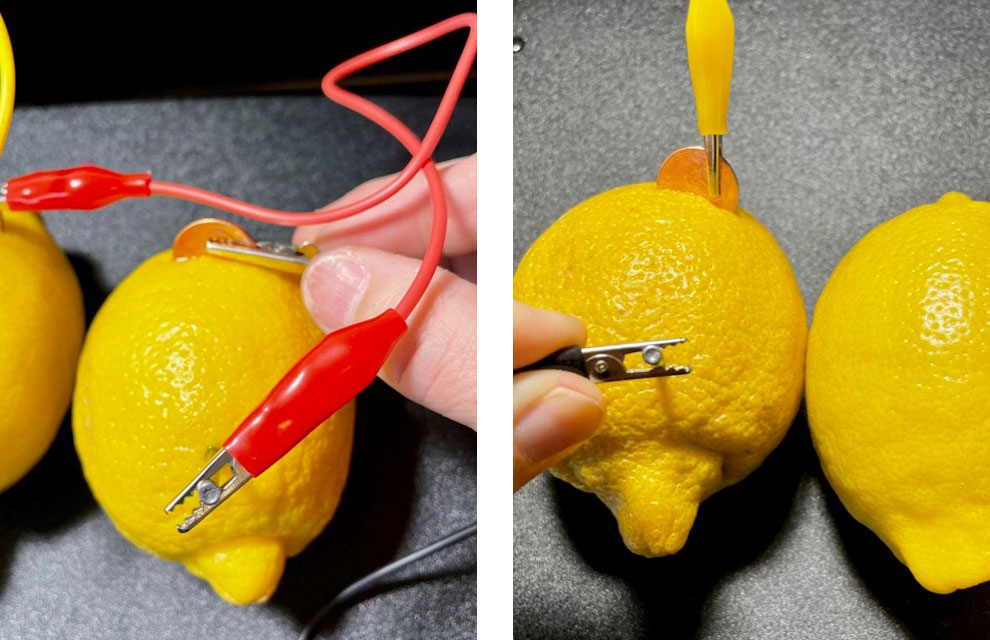
- Push the zinc-galvanized nail into each lemon (4 times).
- Do not have the nail and penny touch inside or outside the lemon.

- Use an alligator clip to connect a nail to the penny of a different lemon.
- Connect all four lemons together with the clips – nail to penny.

- Connect an alligator clip to the last open penny – one end of the clip will be unattached.
- Connect an alligator clip to the last open nail – one end of the clip will be unattached.
- Verify each penny and each nail have a clip attached to them.
- Attach the unused clips to each side of the LED.
- Does it light up?

- A battery contains two different metals that are suspended in an acidic solution. What two metals are used in our lemon battery? Zinc and copper!
- The battery acid is the citric acid found in the lemons. The metal clips on the alligator wires help the electrical current enter and leave the lemon battery. Electrons flow out of the penny, through the wires, into the nail, then move through the acid in the lemon, back out the copper penny, through the wires, into the nail … repeating until it flows through the LED to return back into the lemon and repeat the cycle all over again. That is a circuit!